Welcome to the BLINCYTO® Resource Hub
Treating patients with B-ALL can be challenging but we have made accessing all the information you need about BLINCYTO® in order to optimise your patient outcomes easy with this comprehensive resource hub.
BLINCYTO® is indicated for the treatment of:1
- Minimal residual disease positive (MRD+) B-ALL in patients in complete haematological remission.
- Relapsed or refractory B-ALL.
BLINCYTO® is the first PBS-approved BiTE® immunotherapy1-5
BLINCYTO® is a bispecific T-cell engager (BiTE®) antibody construct that binds to CD19 on the surface of cells of B-lineage origin, and CD3 expressed on the surface of T-cells.1-3 BLINCYTO® mediates the formation of a bridge in the form of an immune synapse between the T-cell and malignant B-cell.1-3
Subsequent lytic proteins released by the T-cell induce target cell death.1-3
BLINCYTO® – an immunotherapy that uses BiTE® technology to engage the body’s own immune system to target malignant cells1-4
Accessing BLINCYTO®
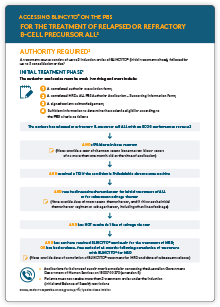
Accessing on the PBS
BLINCYTO® is available on the PBS for the treatment of MRD+ B-ALL and R/R B-ALL via an AUTHORITY prescription.5
BLINCYTO® is the only PBS-listed therapy for the treatment of MRD+ B-ALL.5
Ordering
BLINCYTO® vials MUST be purchased direct from Amgen via the DHL Customer Service Team using a dedicated account set up solely for this purpose.
Dosing and administration1
Use of BLINCYTO® should be restricted to physicians experienced in the treatment of haematological malignancies.

- Because of its short half-life (<3 hours), BLINCYTO® must be adminstered as a continuous IV (cIV) infusion delivered at a constant flow rate using an infusion pump

- A single cycle is 4 weeks of BLINCYTO® cIV infusion
- Each cycle is separated by a 2-week treatment-free interval

- Patients with R/R B-ALL may receive up to a maximum of 5 cycles of BLINCYTO® treatment
- For maintenance therapy, a cycle of treatment of BLINCYTO® consists of 28 days of cIV infusion followed by a 56-day treatment-free interval (maintenance therapy with BLINCYTO® is not covered under the PBS listing)
- Patients with MRD+ B-ALL may receive up to a maximum of 4 cycles of BLINCYTO® treatment




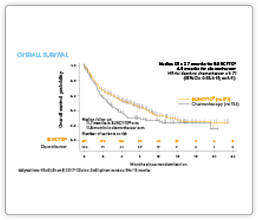
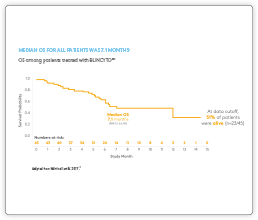
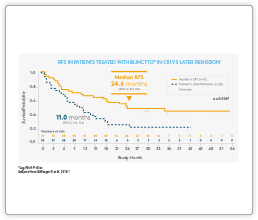
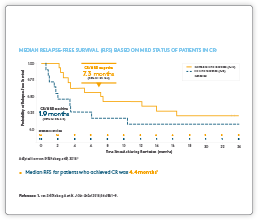
Clinical Data
Please see summaries of the key BLINCYTO® studies below. If you are interested in receiving slides for educational purposes please contact Amgen Med Info here.
Patient Management
Adverse events may be clinically managed with treatment interruption and/or dose adjustments1
BLINCYTO® can be restarted when the AE is resolved or no more than Grade 1 (mild), depending on the type of AE.1
- Grade 4* (life-threatening) neurologic event
- More than one seizure
- Neurologic event leading to treatment interruption that requires greater than a week to resolve
- Grade 3* (severe) neurologic event that occurs at 9 mcg/day dose in patients weighing ≥45 kg or 5 mcg/m2/day dose in those weighing <45 kg leading to treatment interruption
- Any Grade 4* event
Warning: The following have occurred in patients receiving BLINCYTO®:1
- Cytokine Release Syndrome, which may be life-threatening or fatal
- Neurological toxicities, which may be severe, life-threatening, or fatal
- Reactivation of JC viral infection
Interrupt or discontinue BLINCYTO® as recommended if any of these adverse events occur.1
-
Patients should be closely monitored for signs or symptoms of1:
- Cytokine release syndrome (CRS)
- Neurological events
- Infections
- Tumour lysis syndrome (TLS)
-
The following should also be monitored during BLINCYTO® infusion and treated appropriately:1
- Laboratory parameters (including, but not limited to white blood cell count [WBC] and absolute neutrophil count [ANC])
- Alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin
Patient management resources

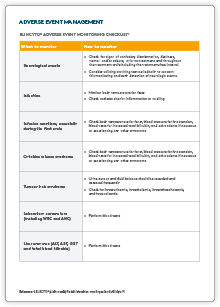





please refer to the BLINCYTO® Approved Product Information at www.amgen.com.au/Blincyto.PI
Patient materials
These guides have been developed for your patients with B-ALL who are treated with BLINCYTO® to help answer some of their questions about their condition and explain what their treatment may be like.







 View the BiTE® Platform video
View the BiTE® Platform video